What is FRACAS?
FRACAS is an acronym for Failure Reporting, Analysis, and Corrective Action System. The term is used to denote a proven process for well-controlled handling of issues that arise in any aspect of business operations. CAPA (Corrective and Preventive Action) processes are used to track and manage issues of concern. A FRACAS is a type of CAPA management system. FRACAS and CAPA processes are employed for management of a wide range of issues such as customer reported incidents, product failures, audit findings, test concerns, manufacturing defects, and many more. These processes are typically part of an organization’s QMS (Quality Management System) and are often tied to compliance requirements. Corrective action management systems provide a well-proven path to support reliability and quality continuous improvement efforts.

In this article, we will look at a few of the most commonly used FRACAS methodologies and provide an overview of each technique’s issue management steps. To learn more details about FRACAS and its benefits, review our in-depth blog post.
Is There a FRACAS Standard?
There is not a single FRACAS standard that is applied across the board. There are many standards that are industry-specific, as well as general guidelines that provide a high-level overview of the implementation of a FRACAS process. The choice of a standard may be made due to industry requirements, compliance needs, corporate objectives, historical reasons, or simply preference. Additionally, how the FRACAS process fits within your organization’s existing quality processes should be factored in when deciding on a FRACAS methodology. The FRACAS issue management process can function as a standalone system but is commonly an integral part of your organization’s quality management system (QMS). Choosing a FRACAS methodology that fits well within your organization’s processes is key to successful issue management.
ISO compliance
Many regulated industries and ISO certified organizations must have a corrective action process in place to meet compliance requirements. Standards such as ISO-9001, IATF 16949 (formerly ISO/TS 16949), AS 9100, and policies that are part of FDA, GMP, and QMS requirements include the need for defined and established FRACAS/CAPA processes.
ISO standards do not impose a methodology that must be followed, but instead allow you to define and implement a system that meets your needs. However, ISO compliancy does require that your process is controlled and followed to your specifications.

MIL-STD-2155 FRACAS Standard
The MIL-STD-2155, entitled Failure Reporting, Analysis and Corrective Action System (FRACAS), establishes the criteria needed to comply with the FRACAS requirement portion of MIL-STD-785. MIL-STD-785, entitled Reliability Program for Systems and Equipment Development and Production, is a broad standard that offers general guidelines as well as specifics for reliability programs that span the product lifecycle. While these standards are military in origin, they are still used across many industries and offer sound guidelines for the FRACAS process.

Accepted FRACAS Process Methodologies
To comply with the needs of ISO, MIL-STD-2155, and other corrective action industry requirements, oftentimes businesses turn to well-defined and well-known process methodologies when implementing FRACAS. There are three commonly adopted techniques that are used across a broad range of industries: 8D, PDCA, and DMAIC.
It is important to note that is it very common for organizations to start with one of these known techniques and modify it to align with their unique needs. By adopting one of these methodologies or a process based on one of these methodologies, you are assured that your FRACAS will meet compliance requirements.
8D: 8 Disciplines of Problem Solving
8D was originally described in publication by the Ford Motor Company in 1987. It was devised as a methodology to ascertain the root cause of a problem, determine an immediate fix, and then define and implement a long-term solution to prevent the issue from reoccurring. For products having issues with defects, the 8D process provides a clear approach for issue management to improve quality and reliability.
8D is often used by quality professionals and is a widely accepted practice in the automotive sector. Because of its sound principles, its use extends to a wide range of industries including aerospace, medical, healthcare, and manufacturing. 8D has become one of the most well-known and established processes for problem solving.
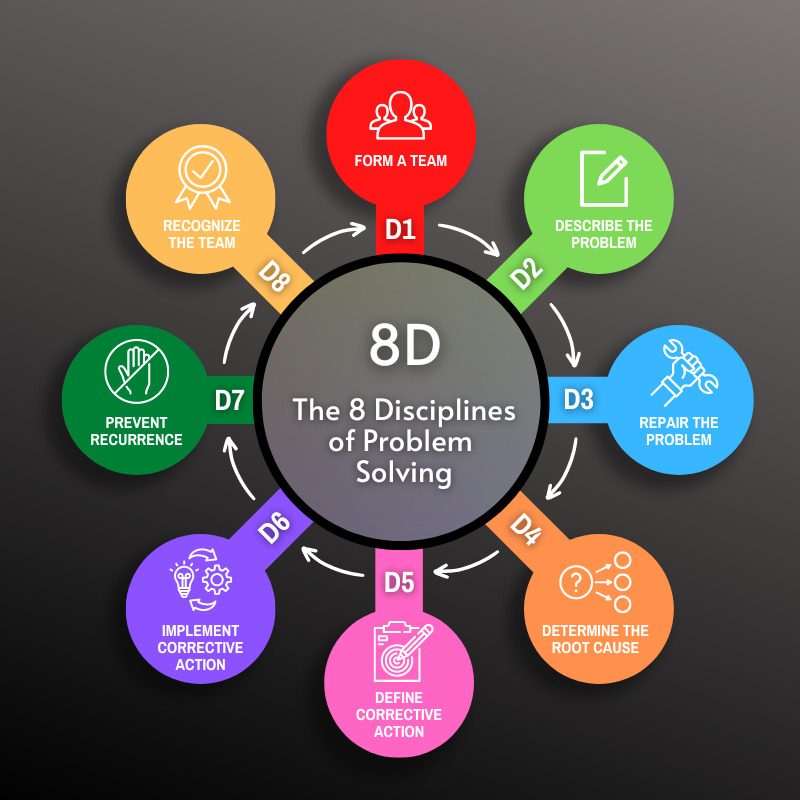
The steps of the 8D process are:
D0: Prepare and Plan
The D0 step is not always considered as part of the main 8D process but is noted as a good starting point. Step D0, Prepare and Plan, entails gathering team feedback before initiating the core 8D process. Ask questions about needs and requirements, gather knowledge from team members who may have 8D experience, and understand goals and objectives. The D0 step can help determine if the 8D process is the best approach to solve the problem.
D1: Form a Team
In Step D1, you identify and form the team that will participate in the 8D process. A cross-functional team is paramount and will ensure successful problem resolution. The make-up of your team depends on your organization and is not a one-size-fits-all approach. You may want design engineers, QA personnel, management, reliability engineers, manufacturing engineering, and subject matter experts (SMEs) as part of your team. The most important consideration is to ensure you are involving all key stakeholders in the process. Additionally, it is vital to have a team leader who has ultimate responsibility for the process.
D2: Describe the Problem
Step D2 involves defining the problem that is the focus of the 8D process. Describing the issue in detail for proper resolution is critical. Data should be collected and recorded to provide insight into failure analysis. If causes have been eliminated, this information is equally critical to include in the problem description to help the team effectively use their time during root cause analysis.
D3: Repair the Problem
Step D3 is the application of the short-term repair or fix. Referred to sometimes as the Interim Containment Action (ICA), step D3 lists actions taken to temporarily correct the problem until a Permanent Corrective Action (PCA) is implemented.
D4: Determine the Root Cause
Before a recommended action plan for corrective action can be defined, the true root cause of the problem must be determined in Step D4. Determining the root cause may be a multi-step process itself depending on the complexity of the issue. Also, you may need to verify your root cause findings with additional data collection and analysis processes.

D5: Define Corrective Action
At this point, based on root cause determination from D4, a corrective action plan can be defined in Step D5. In some cases, there may be more than one corrective action solution proposed. In these situations, it may be necessary to weigh the risks and benefits of each potential action based on aspects such as cost, time constraints, and safety risk. It is important to have a detailed recommended action plan including acceptance criteria to establish success.
D6: Implement Corrective Action
Step D6 involves implementing the accepted corrective action plan. A detailed plan is key. You must be sure to communicate to team members what the plan entails, make sure all team members are involved, and assign responsibilities as needed to ensure all tasks are completed. It is also important to include verification in your plan to validate that the plan was properly implemented and fixed the problem.
D7: Prevent Recurrence
An important element of the 8D process is the lessons learned aspect. This is the focus of Step D7. By retaining the knowledge gained and learning from its lessons, you can improve current and future products and processes. Preventing future issues means reviewing current procedures for improvement, developing new work instructions, or updating control plans. By building on the knowledge gained through the 8D process, your overall drive for continual product improvement is ensured.
D8: Recognize the Team
The final step enables you to review the entire process and celebrate the success of a problem solved. Bringing the team together also allows everyone to see the value of the 8D process and to learn how to improve the process moving forward.
PDCA: Plan, Do, Check, Act
PDCA is a four-step process also referred to as the Deming cycle or Deming circle, or the Shewhart cycle. In the 1920’s, a renowned statistician Dr. Walter Shewhart developed a 3-step process for problem solving. Then in the 1950’s, Dr. W. Edwards Deming further developed the 3-step process, added an additional step to create the PDCA cycle, and solidified the 4-step process widely in use today. The efforts were originally developed to improve and maximize production processes during World War II.
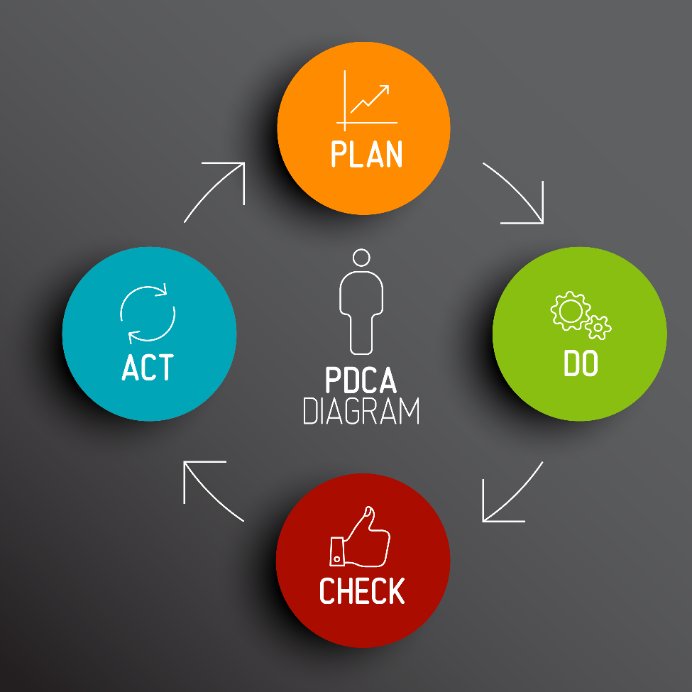
The steps of the PDCA cycle are:
Step 1: Plan
Create a plan to resolve the problem. Make sure the plan is detailed and includes an implementation strategy and success criteria.
Step 2: Do
Implement the plan as delineated. This includes building the team and communicating the plan, performing the steps of the plan, and then finishing by documenting the plan as enacted. This step also includes testing to verify the corrective action plan has achieved the necessary problem resolution.
Step 3: Check
Once implemented, check the plan periodically to verify its success. Using the criteria defined for success, check to make sure the action taken is sufficient. If further or new failures are identified, then make any necessary modifications to the plan.
Step 4: Act
The final step of PDCA is very similar to Step D7 of the 8D process: apply lessons learned for current and future project benefit. The result will be overall product and process improvement and improved reliability and quality across the board.
DMAIC: Design, Measure, Analyze, Improve, Control
DMAIC was also developed by Dr. Deming in the 1950’s and its roots in the PDCA process are evident. The DMAIC methodology has been in consistent use in Six Sigma practices since its inception. The DMAIC five-step process improvement technique is a widely recognized approach for problem solving.

The steps of DMAIC are:
Step 1: Design
The first phase is to define the problem that needs to be fixed, the overall goals you expect to achieve, and the project plan. The Design step encompasses several important aspects, including determining the team members, describing the problem in a detailed manner, defining the business case for this task, and ensuring the goal of the process is understood. Also, as part of this step, a project schedule and overall scope of work should be prepared.
Step 2: Measure
The Measure step is the time for data collection and baseline measurements. You need to determine the current state of your process, its performance metrics, and critical areas that require improvement. At the end of the Measure phase, the team should have a clear understanding of the key performance metrics used to determine success, as well as the current baseline measurements.
Step 3: Analyze
The Analyze step is the same as Step D4: Determine Root Cause of the 8D process. As previously stated, root cause analysis (RCA) may be a multi-step process to zone in on the true underlying problem cause. Without a solid root cause analysis, you can waste time and money implementing solutions that do not fix the problem. It is vital to understand the root cause of an issue by testing and verifying to ensure the correct cause is uncovered.

Step 4: Improve
Now that the problem is understood, create a plan for resolution in the Improve step. The plan is implemented, and verification steps are completed to make sure the process improvement goals have been achieved. By taking time to perform the previous tasks with care – establishing performance metrics goals and assessing root cause – the Improve step has a solid foundation to ensure and verify successful problem resolution.
Step 5: Control
As with each of the process management methodologies, a key element is the retention of lesson learned and their application to current and future products and processes. In the Control step of DMAIC process, the team takes the opportunity to develop a plan to monitor the process to ensure the problem remains resolved. Additionally, it is also critical to update procedures and practices based on knowledge gained during this project.
Relyence FRACAS
Relyence FRACAS offers a fully compliant off-the-shelf software solution for your FRACAS and CAPA requirements. It supports the 8D, DMAIC, and PDCA process methodologies, as well as any custom-tailored methodology desired. It supports your requirements for ISO standards such as ISO-9001, IATF 16949 (formerly ISO/TS 16949), AS 9100, and can help meet requirements that are part of FDA, GMP, and QMS goals. Relyence FRACAS also supports the components of MIL-STD-2155.
It should be noted that while many organizations utilize the well-accepted process methodologies, many tailor these processes for their needs or develop their own process that cater to their unique situation. Relyence FRACAS is a flexible, adaptable, and easily customizable solution that supports these needs.
Additionally, Relyence FRACAS provides a host of powerful features to expand and enhance your corrective action process, resulting in a robust and effective platform for all your FRACAS or CAPA needs.
Check Relyence FRACAS out today. Sign up for a completely free no-hassle trial, schedule a personal demo at your convenience, or contact us to speak to one of our knowledgeable representatives.





